Explore 15 Key Difference between reducing and non reducing sugar
Reducing sugars and non-reducing sugars are two categories of carbohydrates that differ in their chemical properties and behavior. Here are 15 key differences between reducing and non-reducing sugar explained in simple terms for your upcoming exams:
Difference between reducing and non reducing sugar
Reducing sugars have a particular chemical group, an aldehyde or a ketone, that enables them to go through a reduction reaction. This group is absent from non-reducing sugars.
The majority of reducing sugars, including glucose and fructose, are water soluble. Non-reducing sugars, like sucrose, can take more time or effort to dissolve but are still soluble.
Sweetness: When compared to non-reducing sugars, reducing sugars typically taste sweeter.
Reducing Properties: Through their aldehyde or ketone group, reducing sugars can break down specific substances like metal ions or other chemicals. This reducing property is absent in non-reducing sugars.
Oxidation: In a chemical reaction, reducing sugars can undergo oxidation, which means they can lose electrons. Sugars that are non-reducing do not experience oxidation reactions.
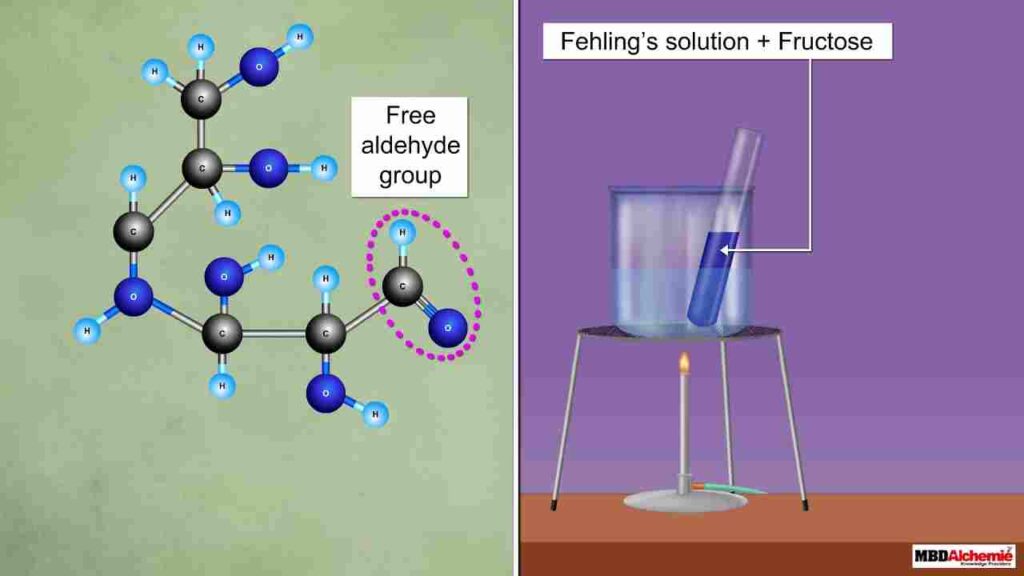
Fehling’s Test: Reducing sugars cause a colour change in Fehling’s solution, going from blue to brick-red. Sugars that are not reduced do not exhibit this reaction.
Benedict’s Test: Similar to the Benedict’s test, reducing sugars result in a positive outcome and a red precipitate. This red precipitate is not produced by non-reducing sugars.
With the addition of water, reducing sugars can be hydrolyzed, or broken down into their individual monosaccharides. Specific enzymes are required for the hydrolysis of non-reducing sugars.
Reducing sugars can be quickly converted into simpler sugars, making them simple for the body to digest. Before they can be digested, non-reducing sugars must be changed into reducing sugars.
Energy Release: Since reducing sugars are quickly absorbed into the bloodstream, they provide quick energy. Energy is released more slowly from non-reducing sugars.
Ordinary Cases: The reducing sugars glucose, fructose, and maltose are typical examples. The sugars sucrose, lactose, and trehalose are non-reducing sugars.
Reducing sugars are frequently present in fruits, honey, and some vegetables, where they act as an energy source. Non-reducing sugars are present in seeds and tubers and are frequently used as storage forms in plants.
Non-reducing sugars can function structurally in organisms by contributing to the construction of cell walls and other protective layers. The main function of reducing sugars is in the metabolism of energy. Non-reducing sugars are less prone to chemical reactions or degradation because they are more stable than reducing sugars.
Fermentation: Sugars that have been reduced can go through the fermentation process, which turns sugars into alcohol or acids. Non-reducing sugars don’t ferment without first being hydrolyzed.
Understanding these differences between reducing and non-reducing sugars can provide insights into their roles, properties, and applications in various fields, such as nutrition, biochemistry, and food science.
Also Read: Explore 15 Key Difference between Microsporogenesis and Megasporogenesis