Explore 15 key Difference between Physisorption and Chemisorption
Physisorption and chemisorption are two types of adsorption processes that occur on the surface of solids. While both involve the adhesion of molecules to a surface, they differ in several key aspects. Here are 15 difference between physisorption and chemisorption, explained in simple terms for your upcoming exams:
Difference between Physisorption and Chemisorption
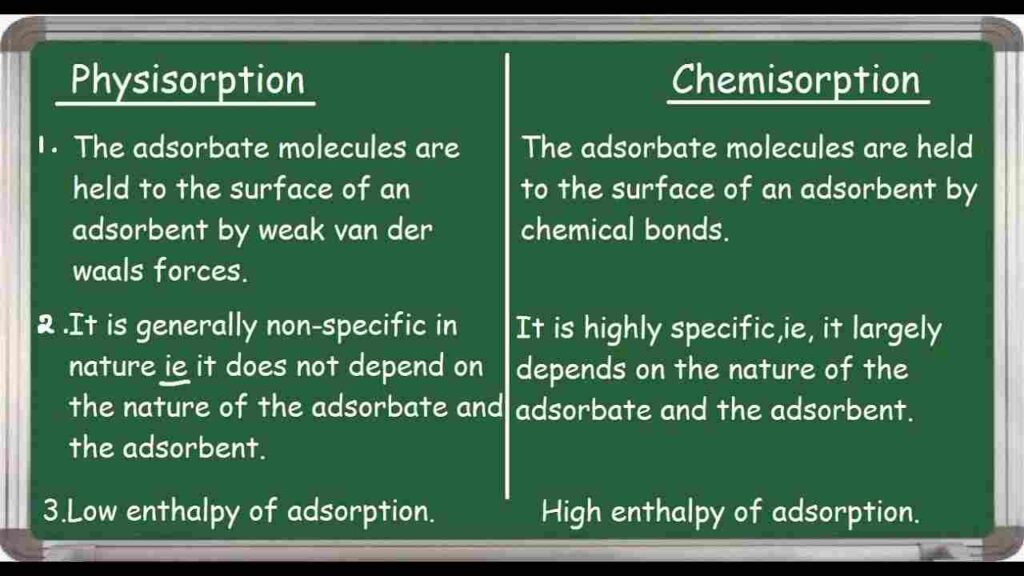
Physical attraction or weak van der Waals forces between the adsorbate (molecules or atoms) and the solid surface are referred to as physisorption. On the other hand, chemisorption entails the creation of chemical bonds between the adsorbate and the atoms on the surface.
Strength of interaction: In comparison to chemisorption, which involves stronger chemical bonds, physisorption interactions are relatively weak.
Chemical bond formation: Physisorption does not involve the creation or breakage of new chemical bonds between the adsorbate and the surface, whereas chemisorption does.
Specificity: Physisorption lacks specificity, allowing adsorbate molecules to adhere to the surface in any way. As the formation of bonds depends on specific chemical characteristics or functional groups, chemisorption is typically more specific.
Chemisorption typically needs an activation energy to get past the energy barrier that prevents the formation of bonds, whereas physisorption doesn’t.
Reversibility: Adsorbate molecules can be easily desorbed from a surface by decreasing surface area or raising temperature, making physical adsorption easily reversible. Since the formation of chemical bonds is more stable and difficult to break, chemisorption is frequently irreversible.
Temperature dependence: Adsorption generally increases with decreasing temperature, and physisorption is typically influenced by temperature changes. Temperature variations have less of an impact on chemisorption.
Low surface coverage is a typical outcome of physisorption because the molecules that adsorb the substance adhere to the surface only loosely. The formation of powerful chemical bonds during chemisorption results in greater surface coverage.
Surface area: Due to weak forces of attraction, physisorption takes place over a larger surface area. Chemisorption takes place on a smaller surface area because it uses particular bond formation sites.
The Freundlich adsorption isotherm, which outlines the connection between adsorbate concentration and surface coverage, governs physisorption. Chemisorption adheres to the Langmuir adsorption isotherm, which takes surface monolayer formation into account.
Adsorption energy: Physisorption requires less energy to attach or detach the adsorbate because it has a lower adsorption energy. Chemisorption requires more energy for attachment and detachment due to its higher adsorption energy.
Physical characteristics of the surface, such as surface roughness or polarity, have an impact on physisorption. The surface’s and the adsorbate’s chemical characteristics affect chemisorption.
Adsorption rate: Because there are weak forces of attraction involved, physisorption happens quickly. Due to the need for the creation of chemical bonds, chemisorption is a slower process.
Applications: Gas storage, chromatography, and catalyst support are three areas where physisorption is frequently used. Chemical sensors, surface modification, and catalysis are all areas where chemisorption is used.
Selectivity: Because different molecules can adsorb on the surface, physical sorption is typically not selective. Because only certain chemical reactions can take place between the adsorbate and the surface, chemisorption can display selectivity.
The degree of interaction, bond formation, specificity, reversibility, temperature dependence, surface coverage, surface area, adsorption isotherm, adsorption energy, surface properties, and rate of adsorption are difference between Physisorption and Chemisorption.
Also Read: Explore 15 Difference between dissolution of partnership and dissolution of firm