Explore Key 15 Difference between Globular and Fibrous Protein
Globular and fibrous proteins are two major types of proteins found in living organisms. While both serve important roles in the body, they differ in their structure, function, and overall characteristics. Here are 15 key difference between globular and fibrous protein, explained in simple words that will help you to understand in simpler way for your upcoming exams:
15 Difference between globular and fibrous protein
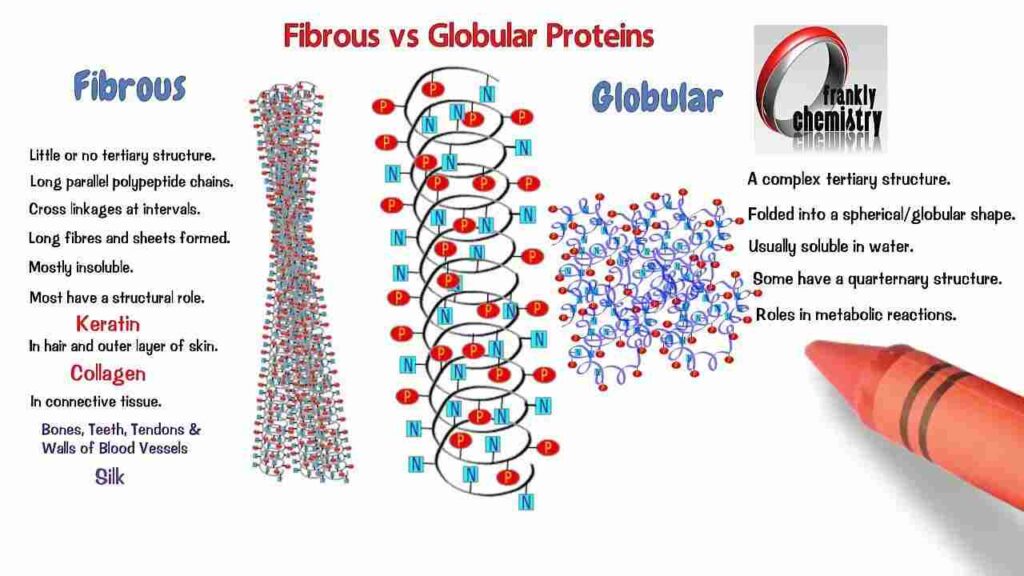
Structure: Fibrous proteins have long, filamentous shapes like fibres or rods, whereas globular proteins have a spherical or globular shape.
Globular proteins are typically soluble in water and other aqueous solutions, whereas fibrous proteins are either insoluble completely or only partially soluble in these same media.
Function: While fibrous proteins are more frequently involved in structural support and mechanical processes, globular proteins frequently play dynamic roles in metabolic reactions, signalling, and transportation.
Because of their compact structure and higher degree of flexibility, globular proteins can go through conformational changes. Fibrous proteins, on the other hand, have a structure that is more rigid and reliable.
Globular proteins are made up of a range of different amino acids, including polar, nonpolar, acidic, and basic residues. A small number of amino acids, including glycine, proline, and hydrophobic amino acids, are frequently found in fibrous proteins.
Hydrophilicity: Globular proteins can interact with water molecules because they have hydrophilic (water-attracting) amino acids on their surface. Hydrophobic (water-repelling) amino acids are present in fibrous proteins, which renders them insoluble in water.
Globular proteins fold into intricate, three-dimensional structures with pockets and clefts inside. Proteins that are fibrous typically fold repetitively in a simpler manner.
Globular proteins frequently have enzymatic activity, which they use as a catalyst in biochemical reactions. Enzymatic activity is typically absent in fibrous proteins.
Transport: Globular proteins take part in processes of active transport, such as the movement of ions or molecules across cell membranes. Structures like tendons, ligaments, and connective tissues often contain fibrous proteins.
Globular proteins frequently have unique binding sites that interact with different molecules, such as receptors, cofactors, or substrates. In general, defined binding sites are lacking in fibrous proteins.
When compared to fibrous proteins, which are typically bigger and longer in size, globular proteins tend to have lower molecular weights.
Protein cleavage by proteolytic enzymes is more likely to occur with globular proteins because of their exposed and accessible regions. Fibrous proteins are less prone to enzymatic degradation and have more resistant structures.
Globular proteins’ compact structure results in a surface area that is greater than their volume. Due to their elongated shape, fibrous proteins have a higher volume-to-surface area ratio.
Globular proteins frequently participate in gene regulation and regulate different cellular processes. The main factor contributing to the mechanical stability and strength of tissues is fibrous protein.
Examples: Amylase and haemoglobin are two examples of globular proteins, while collagen, keratin, and elastin are examples of fibrous proteins.
It is easier to appreciate the diverse roles that globular and fibrous proteins play in living organisms‘ intricate biological systems when we are aware of these important difference between them.
Also Read: Explore 15 Key Difference between Contact and Non Contact force