Understand the Ten Difference between isothermal and adiabatic process
Discover the intriguing realms of thermodynamics as we unravel the difference between isothermal and adiabatic process. Prepare to delve into the captivating world of thermal dynamics and explore the unique dances of these two fascinating phenomena.
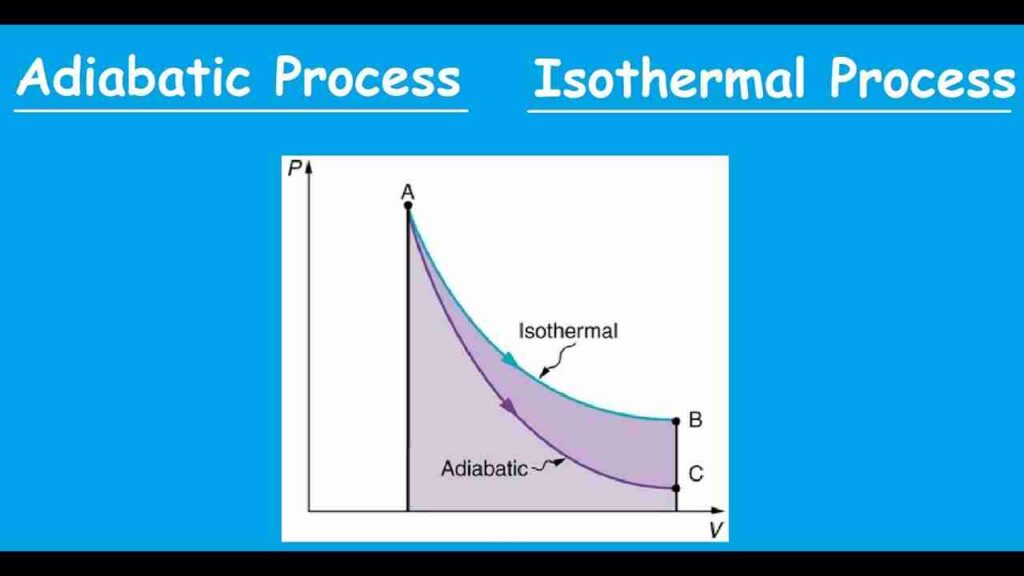
Difference between isothermal and adiabatic process
Two frequently used terms in thermodynamics that describe how a system behaves when its state changes are isothermal and adiabatic processes. There are a number of significant differences between the two, despite the fact that both involve changes in temperature, pressure, and volume.
Heat Transfer: The existence or absence of heat transfer is the key distinction between isothermal and adiabatic processes. An adiabatic process is defined by the lack of heat transfer, whereas an isothermal process involves heat transfer with the environment to maintain a constant temperature.
Temperature Change: During an isothermal process, the system’s temperature doesn’t change at all. In contrast, as the system is compressed or expanded during an adiabatic process, the temperature can change.
Work Performed: During an isothermal process, the system’s work is directly inversely correlated with the temperature change. On the other hand, the work completed in an adiabatic process is dependent on the system’s initial and final states and has no direct relationship to temperature.
Heat Capacity: Because isothermal processes involve heat transfer, the system has a heat capacity that is not zero. Adiabatic processes, like an ideal gas, do not involve any heat transfer and may even have no heat capacity.
Energy Conservation: Because heat transfer is necessary to maintain a constant temperature, an isothermal process needs to be energy-efficient. As no heat is transferred, adiabatic processes, in contrast, are frequently regarded as energy-conserving.
Efficiency: Because isothermal processes can use heat transfer to generate work, they may be more effective than adiabatic ones in some applications, like heat engines. On the other hand, because there is no heat transfer during adiabatic processes, less work may be accomplished.
Isothermal processes require a slow compression or expansion of the system to keep the environment at a constant temperature. Adiabatic processes can happen quickly and could cause a big temperature change.
Also Read: Understand the 10 Key Difference between hotel and restaurant
Reversibility: Because the temperature stays constant throughout the process, isothermal processes are regarded as being reversible. Due to the possibility of energy loss from a temperature change, adiabatic processes are typically irreversible.
Real-World Applications: While adiabatic processes are frequently found in engines, compressors, and turbines, isothermal processes are frequently used in refrigeration and air conditioning systems.
Mathematical Models: While adiabatic processes can be accurately described by additional mathematical models, such as the adiabatic index, isothermal processes can be modelled using the ideal gas law alone.
While there are some similarities between isothermal and adiabatic processes, they differ greatly in terms of heat transfer, temperature change, work done, and energy conservation. Foreseeing the behaviour of thermodynamic systems and creating effective processes for various applications requires an understanding of these differences.