Understand the TEN Difference between molecularity and order of reaction
Welcome, curious reader, to the fascinating realm of chemical kinetics! Today, we embark on an illuminating journey to unravel the enigmatic difference between molecularity and the enigmatic order of reaction. Brace yourself for an intellectual odyssey!
Difference between molecularity and order of reaction
It’s common to hear the terms “molecularity” and “order of reaction” used interchangeably when talking about chemical reactions. But in reality, they refer to two distinct aspects of a reaction, each with their own special traits. For properly analysing and predicting the behaviour of chemical reactions, it is essential to comprehend the distinction between molecularity and order of reaction.
The number of molecules involved in a single step of a chemical reaction is referred to as the reaction’s molecularity. This is referred to as an elementary reaction occasionally. A reaction that only involves one molecule in a single step is known as a unimolecular reaction, whereas a reaction that involves two molecules in a single step is known as a bimolecular reaction, and so on.
The relationship between a reaction’s rate and its reactant concentration is referred to as the order of reaction, on the other hand. In other words, it’s a measurement of how the rate of the reaction is influenced by the concentration of the reactants. By monitoring the rate of the reaction at various reactant concentrations, this can be experimentally determined.
Following the definitions of molecularity and order of reaction, let’s examine some of the main distinctions between the two ideas:
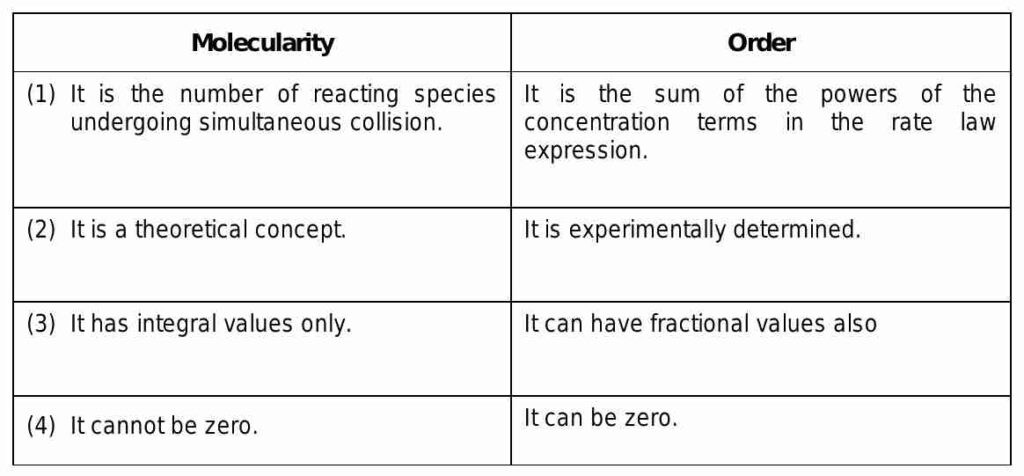
While order of reaction describes the overall behaviour of the reaction, molecularity is a property of a specific step in a reaction mechanism.
In contrast to the experimental determination of reaction order, molecularity is a theoretical concept.
While order of reaction is a fractional value that describes how the rate of the reaction changes with the concentration of the reactants, molecularity is an integer value that describes the number of molecules involved in a single step.
While the order of a reaction is dependent on the reactant concentrations, molecularity is independent of reactant concentrations.
A fundamental characteristic of a reaction mechanism is molecularity, whereas the order of the reaction can change depending on the circumstances of the reaction.
The rate law for an elementary reaction can be predicted using molecularity, whereas the rate law for an overall reaction can be found using the order of the reactions.
If all of the steps in the mechanism are elementary reactions, then molecularity can be used to determine the overall order of a reaction.
While order of reaction cannot be used to pinpoint the rate-determining step in a reaction mechanism, molecularity can.
Also Read: Understand the Ten Difference between impulse and reaction turbine
While order of reaction is related to the rate of the reaction, molecularity is related to the stoichiometry of the reaction.
While order of reaction has real-world applications in chemical engineering and industrial processes, molecularity is purely a theoretical concept.
While order of reaction and molecularity are related, they are separate concepts that cover different facets of a chemical reaction. It is essential to comprehend these variations in order to properly analyse and forecast the behaviour of chemical reactions.